The Galvanic Series and Corrosion Coupons
Galvanic Corrosion is a type of corrosion that takes place when dissimilar metals come into contact with each other in the presence of an electrolyte, which is why it is sometimes referred to as dissimilar metal corrosion. When using corrosion coupons as an internal corrosion monitoring tool galvanic corrosion should be avoided as it will create variability and inaccurate corrosion rates.
Sources of galvanic corrosion can show up in subtle or unexpected ways. For example, a common mistake is to install a copper corrosion coupon up stream of a carbon steel coupon in a corrosion coupon rack. When the copper corrosion coupon corrodes, copper ions dissolve into the water and can plate on the steel coupon, galvanic attack will then accelerate corrosion of the steel coupon. This can lead to significantly higher steel corrosion rates.
Three conditions are required for galvanic corrosion to take place:
Copper deposited on a steel corrosion coupon in a laboratory demonstration.
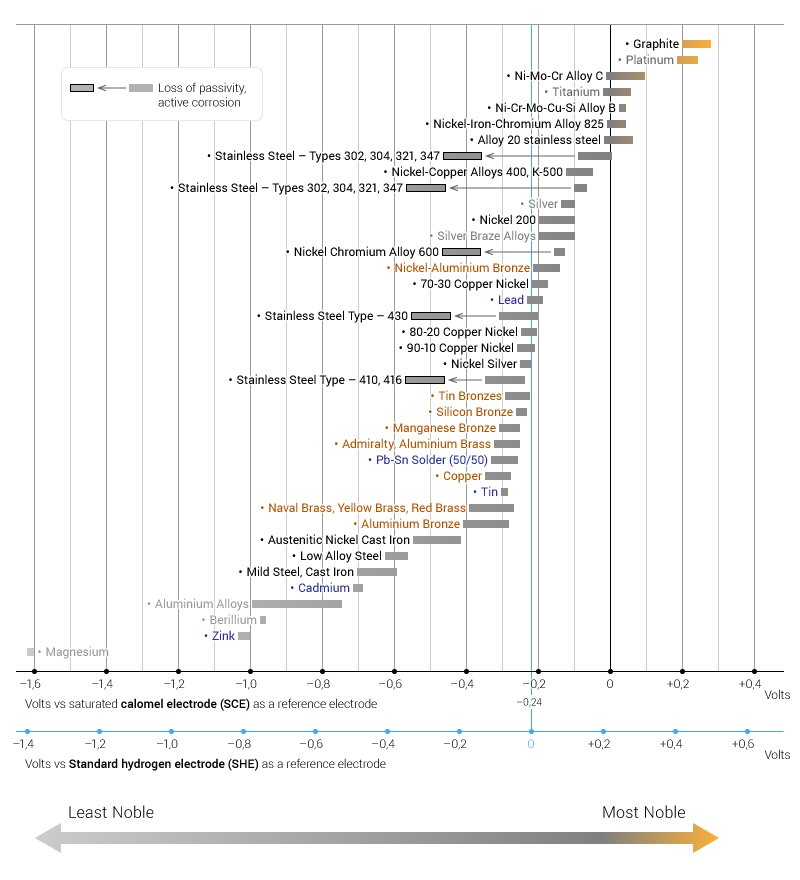
1. There must be a voltage potential difference between the two alloys – see the tables below.
2. An electrolyte such as water must be present.
3. There must be an electrical path.
To avoid galvanic corrosion one or all these conditions must be avoided. In the context of internal corrosion monitoring there is often water or some other electrolyte present, so we focus on the first and third condition.
As it relates to condition three all corrosion coupons should be electrically isolated from any metal alloy in the system. This is typically accomplished by using coupon holders and hardware made from nonconductive materials such as PVC, Nylon, and Teflon. If the operating conditions require a steel or stainless-steel coupon holder the coupon must be isolated from the holder using a nonconductive insulator, typically made from Nylon (up to 180F), Teflon (up to 400F), or Ceramic (1700F).
In some cases, condition one is avoided simply because corrosion coupons of dissimilar metals are not used simultaneously in the system. In systems where corrosion coupons of various metals are in use, such as in a coupon rack, special care should be taken to ensure the coupons are installed in order of nobility. The least noble alloy should be in the most upstream location in the rack, and the most noble alloy furthest downstream closet to the outlet on the coupon rack. The Association of Water Technology (AWT) provides the following:
Position: (from direction of flow)
1: Aluminum (least noble)
2: Galvanized
3: Mild Steel
4: Brass
5: Copper
6: Nickel
7: Stainless Steel (most noble)
This order can be confusing and difficult to remember given that a properly installed coupon rack should flow from the bottom up. Said another way position 1 should typically be at the bottom of the rack nearest the fluid inlet, flowing up to coupon holder number 2 and so forth. According to AWT installing coupons in the correct order will “prevent the more noble metal or alloy from cathodically depositing on the active metal or alloy” and subsequently prevent frustration with variable and inaccurate corrosion rates.
Table 1 from https://www.engineeringclicks.com/galvanic-series/ Note: corrosion coupons should be installed in order from least noble to most noble by direction of flow =>.
Table 2 from https://www.qsl.net/n9zia/electrochemical.html Note: The greater voltage potential between the alloys the greater potential for galvanic attack. Deposits from corrosion coupons upstream can cause galvanic corrosion.